Monochromatic radiation is passed through a gaseous or liquid sample, the light is scattered and detected
| Rotational Raman Spectroscopy | ||||||||
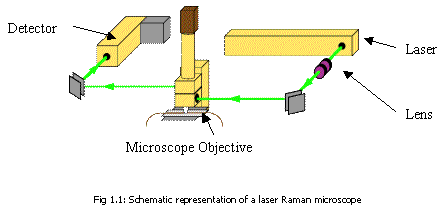
| The Laser The rotational Raman spectrum is inherently weak, so high intensity light and sensitive detectors are required. Lasers produce a narrow, highly monochromatic, coherent beam which can be focused very finely onto a small sample. Continuous lasers routinely have powers of up to several watts. but with good detectors, Raman spectra can be observed even using He/Ne lasers. Rare gas lasers (based on Ar+ or Kr+, for example) which are often used in Raman experiments, can produce light a million times more intense than sunlight. Microscope Objective This focuses incoming laser light onto the sample and collects the scattered light. This outgoing light includes both Rayleigh and Raman scattered light. Detector At the spectrometer incoming radiation is dispersed with a grating, and then detected using diodes, a camera or another type of detector. Detectors may be cooled in liquid nitrogen to reduce thermal noise. | ||||||||
| - The laser produces a narrow beam of light; consequently a spectrum can be obtained from very small samples, or from small areas in larger samples, which allows characterisation of different regions of a surface, for example. - Unlike infrared spectroscopy both incident and scattered radiation are typically at ultra-violet or visible frequencies so glass or quartz optics and sample containers can be used; water is often a feasible solvent. In contrast, IR spectra usually require the use of NaCl or KBr plates. Disadvantages - High-powered lasers may lead to decomposition of the sample. - Florescence occurs when an electronically excited molecule decays back to the ground state spontaneously. Such radiation can completely swamp the weak Raman signal. Using a different laser frequency can generally solve this problem. | ||||||||
Interpreting the Spectrum
The first applet uses the Rigid Rotor model to simulate the Rotational Raman Spectrum of an hypothetical molecule,
| Atom | |
| Mass | 1u |
| Nuclear Spin | 1/2 |
| Bond Length | 100pm |
| zero |
For Part one answer all questions using the Rigid Rotor Model.
Lines are labelled by the J value of the level from which the transition takes place. The first Stokes line is a transition from the rotational ground state, Stokes(0). The first Anti-Stokes line takes place from the energy level with J = 2 to the ground state, Anti-Stokes.