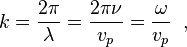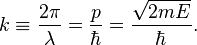Wavenumber
Wavenumber is in the physical sciences a property of a wave proportional to the reciprocal of the wavelength. It can be defined as either
- the number of wavelengths per unit distance, that is, 1/λ where λ=wavelength,
- or alternatively as 2π/λ, sometimes termed the angular wavenumber or circular wavenumber or, simply wavenumber.
In wave equations
In general, the angular wavenumber k, the magnitude of the wave vector, is given by
where ν (Greek letter nu) is the frequency of the wave, ω = 2πν is the angular frequency of the wave, and vpphase velocity of the wave. is the
For the special case of an electromagnetic wave in vacuum, where vp = c, k is given by
where E is the energy of the wave, ħ is the reduced Planck constant, and c is the velocity of light in vacuum.
For the special case of a matter wave, for example an electron wave, in the non-relativistic approximation:
Here p is the momentum of the particle, m is the mass of the particle, E is the kinetic energy of the particle, and 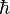 is the reduced Planck's constant.
is the reduced Planck's constant.
 is the reduced Planck's constant.
is the reduced Planck's constant.In spectroscopy
In spectroscopy, the wavenumber  of electromagnetic radiation is defined as
of electromagnetic radiation is defined as
 of electromagnetic radiation is defined as
of electromagnetic radiation is defined aswhere λ is the wavelength of the radiation in a vacuum. The wavenumber has dimensions of inverse length and SI units of reciprocal meters (m−1). Commonly, the quantity is expressed in the cgs unit cm−1, pronounced as reciprocal centimeter or inverse centimeter, or retemitnec by some, and also formerly called the kayser, after Heinrich Kayser. The historical reason for using this quantity is that it proved to be convenient in the analysis of atomic spectra. Wavenumbers were first used in the calculations of Janne Rydberg in the 1880s. The Rydberg-Ritz combination principle of 1908 was also formulated in terms of wavenumbers. A few years later spectral lines could be understood in quantum theory as differences between energy levels, energy being proportional to wavenumber, or frequency. However, spectroscopic data kept being tabulated in terms of wavenumber rather than frequency or energy, since spectroscopic instruments are typically calibrated in terms of wavelength, independent of the value for the speed of light or Planck's constant.
A wavenumber can be converted into quantum-mechanical energy E in J or regular frequency ν in Hz according to
 ,
, .
.
Note that here wavenumber and the speed of light are in cgs units, so care must be taken when doing these calculations.
For example, the wavenumbers of the emissions lines of hydrogen atoms are given by
where R is the Rydberg constant and ni and nf are the principal quantum numbers of the initial and final levels, respectively (ni is greater than nf for emission).
In colloquial usage, the unit cm−1 is sometimes referred to as a "wavenumber",[1] which confuses the name of a quantity with that of a unit. Furthermore, spectroscopists often express a quantity proportional to the wavenumber, such as frequency or energy, in cm−1 and leave the appropriate conversion factor as implied. Consequently, a phrase such as "the energy is 300 wavenumbers" should be interpreted or restated as "the energy corresponds to a wavenumber of 300 cm−1." (Analogous statements hold true for the unit m−1.)